Varicose vein foam treatment is one of the popular and reliable solutions for resolving unsightly veins in the legs. While extremely rare, developing blood clots from the treated vein is still a possibility, and patients with factor V Leiden are at a higher risk. Because of this, some consider taking an organic dietary supplement called serrapeptase to prevent and treat blood clots and inflammation when having varicose vein treatment.
So what should you know about serrapeptase in patients with factor V Leiden when having foam sclerotherapy treatment? Serrapeptase may break up blood clots and reduce inflammation and infection, but it’s still not a primary treatment option for preventing and treating blood clots after foam sclerotherapy in patients with factor V Leiden. This dietary supplement hasn’t undergone extensive clinical trials to provide conclusive claims.
Can Serrapeptase Resolve Blood Clots on Patients with Factor V Leiden When Having Foam Sclerotherapy?
Serrapeptase, a type of proteolytic enzyme, can serve as a prophylaxis and treatment for blood clots in patients with factor V Leiden when having foam sclerotherapy. This is due to its effects of reducing inflammation and infection and dissolving blood clots.
Clinical studies and anecdotal evidence may have reported promising outcomes of serrapeptase. But these records lack conclusive results on the effects and safety of the dietary supplement in preventing and treating blood clots in patients with factor V Leiden when undergoing foam sclerotherapy treatment.
Serrapeptase is still considered a dietary supplement in the United States, not a medication. It still hasn’t been approved by the FDA for any time of treatment or prescription. Because of this, your healthcare provider might not prescribe them as a primary medication for blood clots and inflammation, though they might recommend it as a supplement.
Foam sclerotherapy is one of the most recommended minimally invasive solutions for resolving spider veins and varicose veins or enlarged and twisted veins in the legs. It’s mostly a safe procedure with fewer side effects and risks compared to other treatments, like varicose vein stripping and ligation.
But in patients with factor V Leiden, the risks of developing blood clots from varicose vein foam treatment is more heightened – this leaves healthcare providers more careful than they already are in performing varicose vein treatments. That’s why they may take a more interventional treatment plan for resolving and preventing blood clots, like prescribing thromboprophylaxis medications.
What You Should Know with Factor V Leiden and Varicose Vein Foam Treatment
Due to its anti-inflammation and anticoagulating properties, serrapeptase became a candidate for preventing and resolving blood clotting in patients with factor V Leiden following foam sclerotherapy. To know more about the interaction of this proteolytic enzyme, patients you might want to learn more about factor V Leiden and the effects of foam sclerotherapy on patients with this condition.
1) What is Factor V Leiden?
Factor V Leiden is a mutation involving increased chances of abnormal blood clots developing, usually in the legs or lungs. This condition is caused by genetic factors, where a patient may have inherited a copy or two of the defective gene. Inheriting this gene from both of your parents significantly heightens your risk of experiencing more abnormal blood clots.
The factor V or F5 gene is responsible for controlling coagulation proteins in your body, called factor 5 – this enzyme helps form blood clots to reduce bleeding during an injury. Mutations in your F5 genes cause changes in the structure of the proteins they produce. This makes patients with factor V Leiden develop blood clots faster and easier than normal.
While this mutation causes an increased risk of developing abnormal blood clots, factor V Leiden doesn’t always cause health problems or complications in most people. This allows them to live as healthily as possible. But patients who’ve developed these blood clots are at risk of getting diagnosed with long-term health conditions, which may even be life-threatening. Some of these risks include deep vein thrombosis (DVT) and pulmonary embolism (PE).
Factor V Leiden may manifest in both men and women – women who have this mutation may be at risk of developing blood clots when pregnant or taking some HRT medications. Patients should take anticoagulant medications or blood thinners to reduce their chances of developing blood clots, avoiding serious complications.
2) Risks of Varicose Vein Foam Treatment on Patients with Factor V Leiden
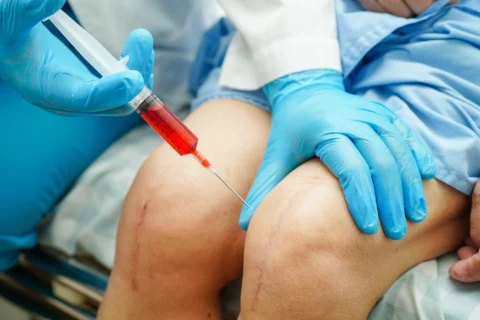
Varicose vein foam treatment or foam sclerotherapy is a solution for resolving varicose veins. It involves injecting a sclerosant into the affected vein, causing scarring and eventually closing the vein. The blood reroutes to other healthier veins while the treated vein collapses and dissolves.
Blood clots from foam sclerotherapy only happen rarely, with an incidence of deep vein thrombosis at 0 to 5.7%. This complication is caused by blood coagulation, as the solution irritates the lining of the blood vessel and closes it down. This usually happens only under the hands of inexperienced injectors without proper injection technique and the use of sclerosant.
But for patients with factor V Leiden, the risk of blood clots may increase due to mutated genes. The development of blood clots can give patients risks of deep vein thrombosis or pulmonary embolism. That’s why healthcare providers performing foam sclerotherapy or other varicose vein treatments in patients with this mutation take extra precautionary measures.
Why Serrapeptase Is a Candidate for Preventing and Resolving Blood Clots
Serrapeptase is one of the candidates for preventing and resolving blood clots when having foam sclerotherapy in patients with factor V Leiden. While there are pieces of clinical and anecdotal evidence of its effects on health, it’s still not FDA-approved as a primary medication to avoid or resolve blood clots. But patients may consult their healthcare providers on taking this as a dietary supplement due to its benefits:
1) What Serrapeptase Can Do
Serrapeptase is a proteolytic enzyme that helps the body break down protein into amino acids, its smaller components. Experts source this from a silkworm’s digestive tract, which they use for digesting and dissolving their cocoon when they’re emerging moths.
Serrapeptase is widely used in Japan and Europe to ease pain and inflammation from trauma, surgery, and other conditions. In the United States, it’s considered a dietary supplement with a wealth of health benefits, like:
- Reducing inflammation
- Preventing the growth of biofilms and other infections
- Reducing swelling
- Speeding up the healing process
- Reducing edema
- Soothing pain
- Promoting normal blood clotting
These serrapeptase benefits made it a candidate for prophylaxis and treatment for blood clots in patients with factor V Leiden mutation after foam sclerotherapy. While these effects greatly help ease inflammation and pain, serrapeptase still offers no conclusive studies for resolving blood clots as a complication from varicose vein foam treatment.
2) What Research Says About Serrapeptase and Blood Clot Resolution
As mentioned, serrapeptase has already been used widely, even in clinical practices. Pieces of clinical and anecdotal reports have validated its positive results, seen in vitro and in vivo tests.
Serrapeptase has been a candidate for preventing and treating blood clots after foam sclerotherapy in patients with factor V Leiden due to its fibrinolytic activity. A clinical trial has studied these effects on in vitro trials.
The tests reported prevention in blood coagulation at 150 U/mL, with 96.6% of blood clot lysis at 37°C after 4 h at 300 U/mL. But these are only in vitro tests, so they might not provide conclusive results yet for being prescribed to patients with factor V Leiden after foam sclerotherapy.
A review of numerous clinical trials has assessed the potential of serrapeptase for therapeutic potential, exploring literature and scientific details on the application of this enzyme. These records come from:
- Controlled in vitro clinical studies
- Uncontrolled pre-clinical in vitro studies
- In vivo clinical studies
- Anecdotal reports
The review has validated the enzyme’s potential for therapeutic applications due to its fibrinolytic, analgesic, anti-inflammatory, anti-edemic, and anti-biofilm effects, reporting that the studies offer strong evidentiary support.
These effects may provide a solid basis for using serrapeptase as prophylaxis and treatment for a blood clot after foam sclerotherapy in patients with factor V Leiden. However, the review still stressed the need for more clinical trials.
After reviewing existing literature on the subject, the researchers have concluded that significant effects of serrapeptase still need scientific data. Larger and more detailed clinical trials should detail the enzyme’s mechanism of action, tolerability, and safety. Serrapeptase has been reported to result in controversial and negative effects, including mild to moderate adverse reactions.
Recommended Treatment Plan for Varicose Veins on Patients with Factor V Leiden

Serrapeptase offers promising results in preventing and resolving complications from foam sclerotherapy and other varicose vein treatments in patients with factor V Leiden. But on top of not having FDA approval, there are still no conclusive trials that test the significant effects of these enzymes.
Serrapeptase is also considered a dietary supplement in the United States, so it might not be used as primary medication. But you may consult your healthcare provider in taking these supplements. For keeping varicose vein treatment in patients with factor V Leiden, they might rely on these solutions, as reported in clinical trials:
1) Venous Duplex Ultrasound Test Before and After Treatment
Before varicose vein treatment, healthcare providers must first assess the affected vein via venous duplex ultrasound test, as reported in a review. This allows them to determine if a patient’s varicose veins are a case of leaky or faulty valves, its usual cause.
Ultrasound imaging can also rule out blood clots or deep vein thrombosis, which may also be associated with varicose veins. If a patient tested positive for blood clots, they’d be recommended a different treatment plan.
Even after varicose vein treatment, healthcare providers will still perform imaging tests to observe if your procedure might have resulted in blood clots. Patients with factor V Leiden mutation must ensure getting these tests to detect possible problems immediately.
2) Varicose Vein Treatments
After ultrasound imaging tests, medical professionals advise varicose vein treatments on affected veins negative for DVT and any other blood clots, as reported in the case reviews. These treatments include:
- Sclerotherapy
- VenaSeal
- Endovenous laser or radiofrequency ablation therapy
- Compression therapy
3) Anticoagulant Medications
Patients with factor V Leiden and other thrombophilic conditions were prescribed thromboprophylaxis medications following sclerotherapy treatment, as reported in a clinical trial. This is to avoid possible complications linked to their conditions, like DVT and the development of other blood clots. The results reported no cases of symptomatic DVT or PE, and the images from venous duplex ultrasound also came in clear.
A review of varicose vein cases also recorded prescriptions of anticoagulants like warfarin or enoxaparin on patients testing positive for DVT or any other blood clots. The results showed resolution with these medications and compression therapy, although there are possibilities of recurrence. The study has concluded that treating faulty veins due to DVT should be beneficial once the thrombus has been dissolved and the venous flow restored.
Resolve Varicose Veins with Highly-Trained Professionals at Vein Center Doctor

Foam sclerotherapy is a reliable varicose vein treatment, but patients with factor V Leiden have a greater risk of blood clots due to mutated coagulation factors. While serrapeptase offers promising results in dissolving blood clots and reducing inflammation, it’s not recommended as primary prophylaxis and treatment of these events post-treatment. A reliable practitioner will provide a treatment plan to remove varicose veins, even with factor V Leiden.
Vein Center Doctor is one of the most reliable vascular centers in New York and New Jersey for treating spider veins, varicose veins, and other related diseases. Our highly-trained medical professionals will provide a customized treatment plan to help resolve your varicose veins, even with factor V Leiden mutation or any other conditions. Get started in improving your quality of life without varicose veins today, and book an appointment with us.