Varicose veins are common, especially in the lower leg. The reason for these enlarged, overfilled veins to be visible in the skin is because of the damage that occurs on the veins, causing irregularity in blood flow, called saphenous vein reflux. Fortunately, there are many options that can be used to eliminate these unsightly veins, and it doesn’t always have to be surgical. One example of non-surgical varicose vein treatment is endovenous laser ablation.
So when did endovenous laser ablation become FDA approved? Since 2002, endovenous laser ablation has been approved by the FDA after much studying, research, and clinical evidence. Following this, demands for this varicose vein removal procedure increased because of its well-tolerated and minimally invasive quality.
Living with unwanted veins is a thing of the past, when you can simply schedule a free consultation with Vein Center Doctor and find your ideal solution today.
Endovenous laser ablation (i.e, endovenous laser therapy) is a non-surgical method of treating varicose veins by heating the defective veins using laser fiber technology. The first of its kind is a catheter ablation performed in 1981 by Dr. Melvin Scheinman, which uses high-energy shocks.
Although it has been around since that time, the more improved version called endovenous laser ablation (EVLA) has only gained FDA approval for use in superficial venous insufficiency in 2002.
The Food And Drugs Administration is a regulatory body that certifies the safety and effectiveness of the food and drug products circulating in the market. This approval aims to regulate drugs and products once they’re out for sale.
The scope of the FDA goes beyond food and drugs, as they also cover treatment procedures and medical devices that are consumed or used in the body, such as endovenous laser ablation. Aside from that, FDA approval also includes the approved uses that treatment procedures and medical devices can be used for.
It’s also important to take note that FDA clearance is different from an FDA approval. An FDA approval went through the process of being certified as safe and effective or as a product that has benefits that outweigh the risk. On the other hand, when a product is FDA-cleared, it means that the manufacturer has proven their product to be substantially equivalent to an already existing FDA-approved product in the market.
An FDA approval doesn’t automatically entail that a product or medical device is devoid of risks — it only means that the regulatory agency has determined that the benefit of using the product outweighs the costs or risks. A product or medical device goes through a long process of clinical trials and testing before it’s FDA approved, which may explain why it took a while for EVLA to be approved even if its predecessors had been around for use since 1981.
Endovenous laser ablation is a safe and effective treatment procedure for all age groups, with excellent success rates in the treatment of an enlarged saphenous vein. This is a treatment that’s generally safe with only a very minimal risk of getting complications. However, like any other treatment procedure, a patient may still be vulnerable to rare side effects such as:
Despite the risks and side effects of endovenous laser therapy, it’s important to note that the risk of non-treatment of varicose veins still outweighs the rare risk of having the non-invasive procedure. The EVLT treatment procedure would be generally risk-free if performed by a qualified healthcare provider.
Since the FDA approval of endovenous laser ablation treatment, more patients have demanded it. Unlike its surgical alternatives in varicose veins removal, EVLA has a faster recovery period and low risk of complications.
It’s effective in eliminating varicose veins, but it still depends on how the body responds to the procedure’s attempt to reabsorb the ablated varicose veins back in the body. Aside from that, this procedure also does not guarantee the permanent removal of varicose veins.
Any product, drug, procedure, or medical device seeking FDA approval goes through a five-step process that must be completed to gain FDA approval. The five-step process that must be completed includes the following:
Research for a medical device is also conducted in the lab, which undergoes laboratory and animal testing to ensure the basic requirements for an FDA approval which involve safety. It is then tested on people to confirm its safety and determine its effectiveness.
The FDA then thoroughly reviews the data results from the research before they come up with a decision for approval. The decision for approval and the classification of a medical device for recall depends on the hazards or risk they pose when used, which is shown in the table below:
| Classification for Recall | Definition |
| Class 1 | Use of the product has adverse health consequences |
| Class 2 | Use of the product has temporary or reversible adverse health consequences |
| Class 3 | Use of the product is not likely to cause adverse health consequences |
When the FDA denies approval, it usually provides a response letter which outlines their reasons for the denial. An opportunity for improvement of the subject for approval is given to the company to meet the qualifications for approval required by the FDA.
Endovenous laser therapy is an FDA-approved, non-invasive way to eliminate varicose veins. It has many advantages that cement its position as a preferred non-surgical method of eliminating abnormal veins.
Before fully choosing endovenous ablation as a preferred method to eliminate the affected vein, it’s essential to understand first the basic things about it such as how it works, its benefits, and the instructions before and after the treatment to get the best results out of the treatment procedure.
Endovenous laser ablation uses laser heat to eliminate the enlarged saphenous vein. The heat emitted by lasers targets the abnormal saphenous vein wall. The treated vein then becomes dead tissue that’s naturally absorbed by the body. During the process, the blood is redirected to the nearby healthy veins.
What happens during the endovenous laser treatment procedure is that a patient is given anesthesia by their healthcare provider to help with the pain. Local anesthesia will be administered to numb the great saphenous vein area, while a sedative medication will be offered to help the patient relax during the procedure.
Using ultrasound guidance, the location of the defective saphenous vein would be identified. A small incision in the leg would be made where a catheter would be inserted, targeting the defective superficial veins. A catheter is a tool used to emit laser heat to permanently seal off the damaged vein.
Endovenous thermal ablation is an effective way to eliminate a diseased vein without the need for surgery. Although its effectiveness depends on the bodily response to the treatment, it could be noted that it has the following benefits:
Having the right preparation for endovenous ablation is essential to boost the effectiveness of the EVLT procedure. Before you’re scheduled to have the EVLT procedure, you must ensure that your provider knows the important details from your medical history and current medications taken to avoid compromising the results of the treatment.
Having a thorough discussion of your current medication, symptoms, and health condition is the best way to prepare for your endovenous laser treatment session to ensure that the side effects that you may experience are minimal and manageable.
During the initial consultation with a varicose vein expert, your eligibility for EVLA would be evaluated by a vein specialist. A treatment plan would be created, which may include the instructions on how to properly take care of your incisions during your healing process, such as
Sometimes varicose veins return or don’t fully heal. Ultrasound guidance will be performed by your provider after a week following your treatment session to confirm if the saphenous vein is sealed and healing. Depending on the results, other varicose vein treatment procedures such as ultrasound-guided sclerotherapy or foam sclerotherapy may be recommended for you.
Like any other treatment, endovenous ablation still has its own expected side effects. However, if it’s performed by an experienced licensed vein specialist or provider, then it’s generally safe and the side effects tend to be minimal. Some of the side effects that are associated with EVLA are:
The expected recovery from the side effects is a few weeks following your treatment procedure. If you don’t experience any improvement in the side effects that you’re experiencing, it's best to consult with your healthcare provider.
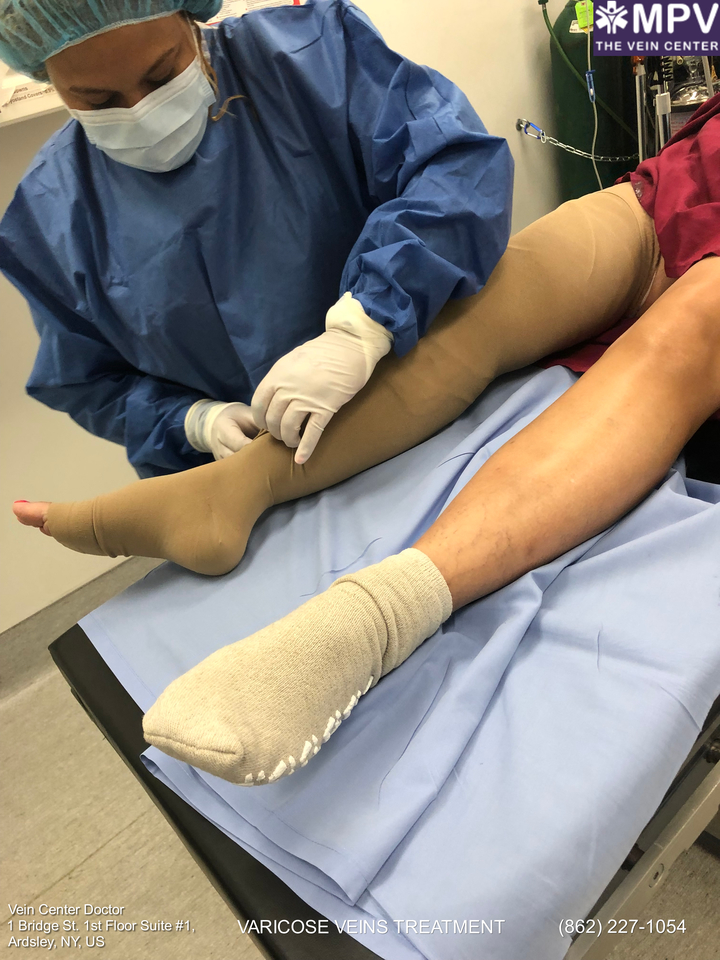
One of the best ways to evaluate the safety and effectiveness of a product is whether or not it has secured FDA approval. An FDA approval may not completely mean that a product is fully safe from risks and side effects, but it does ensure that the benefits of using the product outweighs its risk; and if it does have any risk, it would be generally manageable.
Fully secure the effectiveness and safety of your treatment procedure by only seeking the services of a qualified vein specialist from the best vein clinic. Here at Vein Center Doctor, our team is led by Dr. Rahul Sood to effectively treat your vein problems (i.e, venous ulcer, incompetent perforator veins, venous reflux) or any vein disease that is caused by medical conditions.
Schedule your appointment at Vein Center Doctor and be free from vein pain today.
Find exactly what you need to get rid of your vein-related problems. Dr. Sood and the rest of our team at Vein Center Doctor are ready to help: schedule your free consultation today.
Most Insurance is accepted for treatment